About Us
Over a decade of experience in RWE and 10+ years of hands-on expertise
The GetReal® Institute evolved from two influential programs supported by the Innovative Medicines Initiative (IMI) — the GetReal Project and the GetReal Initiative. These laid the groundwork for our collaborative approach, positioning us as a trusted body focused on facilitating the integration of RWE into healthcare decision-making in Europe.
GetReal Project
The GetReal Project (2013-2017) was established to demonstrate how robust new methods of real-world data (RWD) collection and synthesis could be adopted earlier in pharmaceutical research and development and the healthcare decision-making process..
GetReal Initiative
The GetReal Initiative (2018-2021) continued to drive the adoption of tools, methodologies and best practice to increase the quality of real-world evidence (RWE) generation in medicines development and regulatory/health technology assessment (HTA) processes across Europe.
GetReal Institute
The GetReal® Institute is established as an independent multi-stakeholder, non-profit forum for collaboration in the adoption and implementation of RWE.
Governance
The GetReal Institute is an independent, multi-stakeholder, non-profit platform committed to advancing high-quality, transparent use of real-world evidence across healthcare decision-making. Collaboration, and scientific integrity are at our core. Our founding principle is collaboration across sectors to advance pre-competitive, scientifically robust solutions in RWE.
A strong and transparent governance framework is fundamental to the GetReal® Institute, ensuring accountability, inclusivity, and strategic oversight. At the core of our governance structure is an elected Executive Board of Directors, currently composed of six experienced members who bring diverse expertise in RWE, healthcare policy, and evidence-based decision-making.
Our Approach
The GetReal® Institute is dedicated to advancing the adoption and integration of real-world evidence (RWE) in healthcare decision-making across Europe and beyond. We focus on fostering collaboration, promoting knowledge exchange, and developing practical resources to support the effective use of RWE in regulatory, HTA, and clinical contexts. By engaging a diverse network of stakeholders, we aim to strengthen the role of real-world data in improving patient outcomes and optimising healthcare systems.
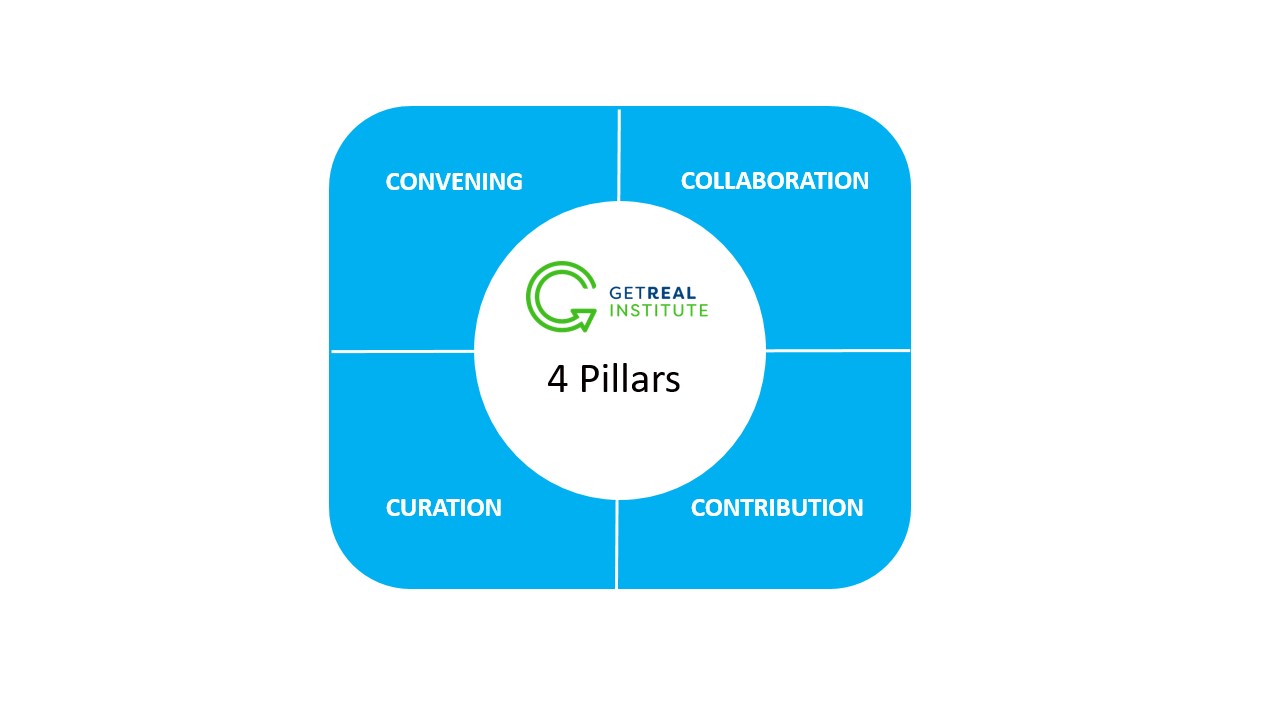
1. CONVENING
The GetReal® Institute fosters a dynamic and inclusive community, bringing together stakeholders from across the evidence generation landscape. By engaging a diverse range of voices, we facilitate the exchange of perspectives and ensure that real-world evidence (RWE) is shaped by broad expertise and experience—making it truly fit for purpose.
2. COLLABORATION
Meaningful progress in evidence-based healthcare requires collective action. The GetReal® Institute offers a structured, independent platform where members engage in productive dialogue, exchange insights, and co-develop innovative methodologies. Through strategic initiatives, collaborative projects, and applied research, we promote best practices and drive the integration of real-world evidence (RWE) into healthcare decision-making.
3. CURATION
Drawing on deep expertise, the GetReal® Institute identifies and shares valuable knowledge and resources. By curating a comprehensive repository of best practices, we equip our members with the insights and guidance needed to navigate the evolving real-world evidence (RWE) landscape with confidence and clarity.
4. CONTRIBUTION
Our commitment to impact is reflected in the development of high-quality resources, guidance documents, and frameworks that enhance the clarity and appropriate application of RWE. Through collaboration with our members and partners, the GetReal® Institute contributes to advancing evidence-based decision-making, ultimately improving patient outcomes and healthcare system efficiency.